
predict the names and states of the products formed predict the reaction type (precipitation, neutralization or gaseous) - record your observations When finished, complete the data sheet by writing the balanced equation for each reaction. Aqueous iron (III) chloride + aqueous ammonium hydroxide.Aqueous sodium chloride + aqueous potassium nitrate.Aqueous sodium carbonate + aqueous cobalt (II) nitrate.Hydrochloric acid + aqueous sodium hydroxide.Aqueous barium chloride + sulfuric acid.Aqueous nickel (II) nitrate + aqueous sodium hydroxide.Hydrochloric acid + solid sodium bicarbonate (just a small scoop).

Aqueous sodium phosphate + aqueous copper (II) sulfate.Aqueous sodium chloride + aqueous silver nitrate.All waste is to be disposed of in the plastic container in the hood! If results are not obtained immediately, give the reaction some time. Perform the following reactions, and record your observations for each on the data sheet.
A good estimate is to use three full dropper squirts of each chemical. Use approximately 3 mL quantities of all solutions.
Most phosphates, carbonates, chromates and sulfides are insoluble (except those of the alkali metals and ammonium). Most sulfates are soluble, except for BaSO 4, SrSO 4, Ag2SO 4, PbSO 4, and CaSO 4. All halides (chlorides etc.) are soluble except for those containing Ag +1, Pb +2, and Hg 2 +2. Hydroxides of alkali metals and NH 4 +1, Ca +2, Sr +2, and Ba +2 are soluble. Alkali metal compounds, acetates, nitrates, and ammonium compounds are all soluble. Always name the cation first, then the anion.Įxample 6: Name the ionic compound Al(NO 3) 3.Ĭation = Al +3 = aluminum anion = NO 3 -1 = nitrate The name of this compound is aluminum nitrate Both the cation and anion must be named.Ģ. The basic rules for writing names of ionic compounds: 1. The numbers of each element present in the compound become subscripts in the chemical formula.Įxample 5: Write the formula for magnesium phosphate.įirst identify the cation and anion in this compound.Ĭation = magnesium = Mg +2 anion = phosphate = PO 4 -3įor a neutral compound, three Mg +2 are needed for every 2 PO 4 -3 The formula of the compound is Mg3(PO4)2 Total charge of all the positive cations must equal the total charge of all the negative anions in the compound. Combine the ions in a ratio that results in the formation of a neutral ionic compound. Determine the formulas and charges on the cation and anion involved in the compound. The basic rules for writing the chemical formulas of ionic compounds: Ionic compounds are formed when positive cations and negative anions are attracted to each Review: Chemical Formulas and Names of Ionic Compounds Balance the equation (to ensure mass conservation).īe sure to include the physical states of all reactants and products in your final equation. If you determine that a reaction will not occur, write “no reaction” after the arrow. If you determine that a reaction will occur, write the correct formula(s) of the products after the arrow. 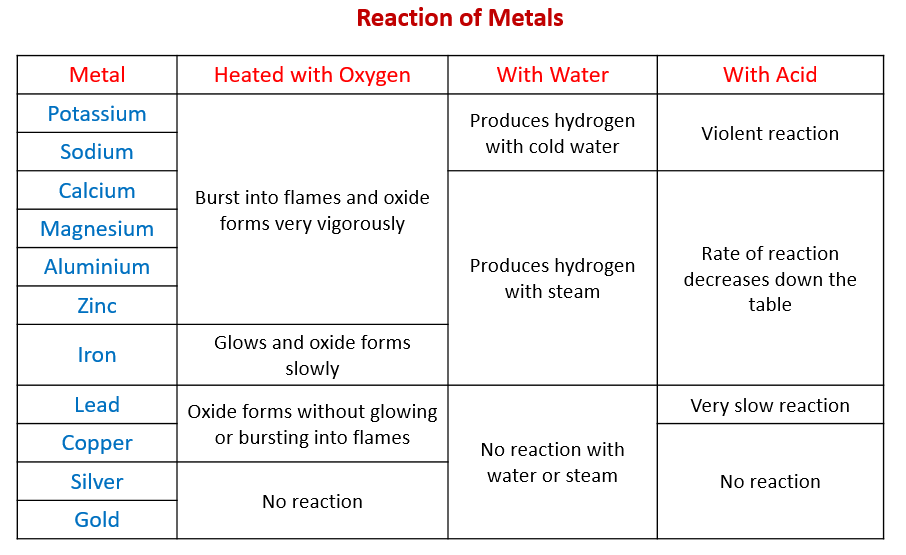
Identify the reaction type – precipitation, neutralization or gas forming.Write chemical formulas for each reactant and place a yield arrow (→ ) after the last reactant.







 0 kommentar(er)
0 kommentar(er)
